|
Pneumothorax Performance | Beating BI-RADS
July 20, 2022
|
|
|

|
|
Together with
|

|
|
|
“Making it simple is not easy.”
|
|
Dr. Alessandro Roncacci, on how hard Affidea is working to make sure its future AI workflows are simple.
|
|
|
|
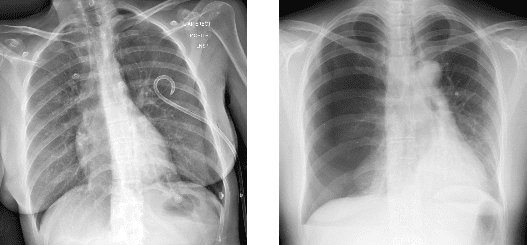
A new Mass General Brigham study highlighted Annalise.ai’s pneumothorax detection solution’s strong diagnostic performance, including across different pneumothorax types and clinical scenarios.
The researchers used Annalise Enterprise CXR Triage Pneumothorax to “analyze” 985 CXRs (435 positive), detecting simple and tension pneumothorax cases with high accuracy:
- Simple pneumothorax – 0.979 AUC (94.3% sensitivity, 92.0% specificity)
- Tension pneumothorax – 0.987 AUC (94.5% sensitivity, 95.3% specificity)
The study also suggests that Annalise Enterprise CXR should maintain this strong performance when used outside of Mass General, as it surpassed standard accuracy benchmarks (>0.95 AUC, >80% sensitivity & specificity) across nearly all of the study’s clinical scenarios (CXR manufacturer, CXR projection type, patient sex/age/positioning).
The Takeaway
The clinical benefits of early pneumothorax detection are clear, so studies like this are good news for the growing number of FDA-approved pneumothorax AI vendors who are working on clinical adoption.
However, this study feels like even better news for Annalise.ai, noting that it is one of the few pneumothorax AI vendors that detects both simple and tension pneumothorax, and considering that Annalise Enterprise CXR is capable of detecting 122 other CXR indications (even if it’s currently only FDA-cleared for pneumothorax).
|




|
|
What Built For the Modern World Means
Find out what built for the modern world means — and why it matters — in this Aunt Minnie profile on United Imaging’s more modern approach to vertical integration, leadership, and culture.
|
|
Microservice Architecture’s Many Benefits
See how cloud-native imaging avoids traditional software’s resource utilization constraints and eliminates unexpected disruptions in this Change Healthcare animation.
|
|
- Kaiser Score Beats Experience: A study in European Radiology found that the “Kaiser score” (KS) breast MRI clinical decision system allows inexperienced radiology residents to achieve better diagnostic accuracy than experienced breast imaging radiologists using MR BI-RADS. Three residents using the KS system and three experienced radiologists using MR BI-RADS interpreted breast MRIs from 80 women (93 lesions, 61 malignant), with the residents achieving higher AUCs (0.842–0.928 vs. 0.723–0.742) and higher inter-reader agreement than the expert rads (kappa 0.579–0.710 vs. 0.531–0.624).
- Caption Health’s CE Mark: Caption Health received a European CE Mark for its Caption AI platform, which uses AI guidance to allow untrained clinicians to perform cardiac ultrasound exams (vs. only sonographers), and could significantly expand access to echo exams. Caption AI gained FDA clearance in 2020 and has since generated solid US momentum, including a high-profile alliance with Butterfly Network and rare NTAP reimbursement.
- Proposing MPFS 2023: Healthcare Administrative Partners provided a complete overview of how CMS’ proposed 2023 Medicare Physician Fee Schedule might impact radiology, headlined by reimbursement cuts (-4.4% to $33.0775 per RVU) that will bring reductions to diagnostic and interventional radiology (-3% & -4%). HAP also revealed positive news, as CMS accepted 10 new radiology-related CPT codes and announced that it would indefinitely delay the AUC/CDS program’s penalty phase.
- CathWorks & Medtronic’s Angio Alliance: Angiography AI company CathWorks and Medtronic expanded their partnership, leading to Medtronic investing an additional $75M in CathWorks (it was already an early investor) and the companies co-promoting CathWorks’ FFRangio System in the US, Europe, and Japan. Depending on certain milestones, Medtronic will have the option to acquire CathWorks for up to $585M. The CathWorks FFRangio System delivers multi-vessel FFR results from routine angiograms, which seems to fit well with Medtronic’s suite of angiography products.
- Ultrasound AI Cystic Hygroma Detection: A new study out of The Ottawa Hospital showed that fetal ultrasound AI models could be used to accurately diagnose cystic hygroma during the first trimester. The researchers trained and tested the AI model using 289 fetal ultrasound images (129 cystic hygroma cases, 160 controls), finding that it identified cystic hygroma with 93% accuracy, 92% sensitivity, 94% specificity, and an AUC of 0.94.
- Reintroducing GE HealthCare: GE Inc.’s separation into three independent companies reached a major milestone, with the unveiling of the companies’ new names and branding. GE Healthcare will be (slightly) renamed GE HealthCare, adopting updated corporate branding that combines its long-established cursive “GE” logo with new “compassion purple” coloring. GE HealthCare will be the first company to first spin-off in early 2023 (Nasdaq ticker: GEHC), followed by GE’s energy business portfolio (renamed GE Vernova) and its aviation business (renamed GE Aerospace) in 2024.
- Handhelds Rival ABUS: A new ScienceDirect study found that handheld breast ultrasound rivals automated breast ultrasound systems (ABUS) for detecting malignant cancer in dense breasts. The two ultrasound systems were used with 592 women, finding that handhelds detected more cystic lesions (1,491 vs. 1,005) and mass lesions (336 vs. 270) than ABUS, but slightly fewer positive/suspicious cases (167 vs. 171). The two ultrasound systems achieved “good” agreement with cystic lesions, “moderate” agreement with solid mass lesions, and “good” agreement with suspicious lesions (k = 0.61-0.62 & 0.57-0.60 & 0.66).
- Annalise.ai’s CE MDR: Annalise.ai announced the CE Mark MDR class IIb recertification of its flagship Annalise CXR Enterprise solution (detects 124 abnormalities in CXRs). Europe’s MDR certification program launched last year, requiring AI products to attain higher risk classifications (IIa, IIb, or III… no longer class I), and setting a 2024 recertification deadline for existing products. However, as of May 2022 only 17% of CE-marked AI products were MDR-recertified.
- Contrast Reinforcements: Contrast shortage relief might be on the way, after the FDA granted Bayer rights to import a limited quantity of its foreign-labeled ULTRAVIST contrast (otherwise the same as its USA-labeled ULTRAVIST) and Fresenius Kabi launched its FDA-cleared generic Iodixanol contrast portfolio (equivalent to GE’s Visipaque). These announcements came shortly after GE Healthcare revealed that it still expects “some ongoing reduced availability” until the global supply chain restabilizes.
- SpinTech MRI’s Series A: SpinTech MRI raised $6.5M in Series A funding that it will use to support the commercial expansion of its FDA-cleared STAGE (STrategically Acquired Gradient Echo) quantitative MRI platform. STAGE is currently deployed at more than 50 institutions, where it’s used by neurologists, radiologists, and researchers to support the MRI-based diagnosis of a range of neurological conditions.
- Affidea & Incepto’s Portuguese Pilot: European imaging giant Affidea announced a pilot collaboration with AI platform company Incepto, beginning with four AI solutions (oncology, neurology, breast imaging) integrated at 14 imaging centers in Portugal. This is one of 10 different AI pilots that Affidea is operating across 10 different countries, although this Portuguese pilot might lead to Affidea expanding the Incepto platform further across Europe.
|
|
- Imaging providers who want to finally #ditchthedisk can now start off with Novarad’s CryptoChart Lite solution, a no-cost version of CryptoChart built for providers transitioning to imaging sharing.
- See how Enlitic’s mission to create time for radiologists led to its shift from point solutions towards enterprise-level solutions that are fundamental to workflow.
- See why radiologist Dr. Eleanna Saloura called Arterys’ Lung AI solution “a fast and reliable second opinion” for chest CT lung nodule analysis and tracking, allowing “more accurate diagnostic and treatment decisions.”
- Trying to figure out how your IT resources can handle increased AI adoption? This Blackford paper details how the cloud is helping radiology organizations scale their computing resources to support multiple AI applications or algorithms.
- Over 9 out of 10 people who should be screened for lung cancer aren’t, and nearly 50% of lung cancer cases are caught in the advanced stages. We know from prostate and breast cancer screening that clear guidelines and increased screening saves lives. But lung cancer screening has been challenging. Riverain strives to make everything about the lungs clearer, so they assembled this resource page for anyone interested in starting or improving their lung screening program.
- Us2.ai’s echocardiography analysis automation solution is being integrated into the EchoNous Kosmos ultrasound platform, creating the most powerful diagnostic “power tool” ever created for the hand-carried POCUS market.
|
|
|
|
|